Summary
Somatic coliphages are a new indicator for monitoring the efficiency of water treatment and purification in the Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption and in Regulation (EU) 2020/741 of the European Parliament and of the Council from 25 May 2020 on minimum requirements for water reuse. For this reason, it will be necessary in the near future to gradually introduce a method for the determination of somatic coliphages in water in some operational or other laboratories. The possibility of using the cultivation procedure in accordance with the procedure in the ČSN EN ISO 10705-2 standard has been addressed since 2019 in the microbiological laboratory of the Department of Water Technology and Environmental Engineering at the University of Chemistry and Technology in Prague. Somatic coliphages were detected in samples of differently polluted waters, specifically in surface waters, wastewaters (effluents from wastewater treatment plants) and grey waters. Selected concentration methods (magnesium hydroxide flocculation and membrane filtration), which must be used for water samples with a low concentration of somatic coliphages (< 3 PFU/ ml), were also tested and verified experimentally. Based on the performed tests, it was found that the membrane filtration method using electronegative filters shows higher efficiency in concentrating somatic coliphages from a water sample than flocculation with magnesium hydroxide.
Introduction
In the last few years, cases of contamination of drinking water with viral agents have been known from water supply practice, whether these were cases associated with the occurrence of enteroviruses, noroviruses, or adenoviruses. These cases were publicized by the press and the professional public. Viruses are generally considered to be more resistant living systems (organisms) to biological and physico-chemical environmental influences than bacteria. Despite the danger of this microorganism, the requirement for its determination within the performed microbiological analyses of water is not taken into account, for example, by Decree No. 252/2004 Coll., as amended, which sets hygienic requirements for drinking and hot water and the frequency and scope of drinking water control. Most viruses cannot be detected by cultivation procedure: molecular biology methods are used instead (e.g., PCR = polymerase chain reaction), which require adequate laboratory equipment, which is expensive. Determination of some types of viruses by cultivation is difficult to perform in an in-house microbiological laboratory because host cells of selected bacterial strains and optimized procedures are required.
Somatic coliphages are currently a much-discussed topic in the field of water quality control, as they show some morphological similarity to human enteric viruses and can be seen as possible indicator organisms for the viral contamination in water. Due to the ever-increasing demands on drinking water quality and the society’s pressure for the reuse of treated wastewater, somatic coliphages were legislatively proposed as new indicators to monitor the efficiency of water treatment and purification. For this reason, it is highly desirable to monitor their presence in certain types of water, especially water intended for human consumption. Somatic coliphages are a group of non-pathogenic bacterial viruses called “bacteriophages”, which infect cells of coliform bacteria (Enterobacteriaceae group) and, in particular, cells of the bacterium Escherichia coli (E. coli). The natural environment of somatic coliphages is, as for E. coli bacteria, the gastrointestinal tract of humans and warm-blooded organisms.
The Directive of the European Parliament and of the Council (EU) 2020/2184 from 16 December 2020 on the quality of water intended for human consumption came into force on 12 January 2021, which is a completely recast amendment to the Directive 98/83/EC. The precondition for the implementation of this Directive into national legislation is the end of 2022 to the beginning of 2023.
The purpose of the Drinking Water Directive is to protect consumer health from the consequences of polluted water use and to ensure the safety of drinking water through defined quality standards, which EU Member States are required to comply with. Among other things, the revision focused on updating the list of drinking water quality indicators, which no longer corresponded to current knowledge. Somatic coliphages are a new indicator that will have to be checked in the operational monitoring of the drinking water treatment process effectiveness if > 50 PFU/100 ml (PFU = plaque forming unit) is detected in the raw water source [1].
Somatic coliphages are also mentioned in the new Regulation 2020/741 of the European Parliament and of the Council (EU) of 25 May 2020 on minimum requirements for water reuse [2]. This regulation should make it easier for the Member States of the European Union (EU) to reuse treated urban wastewater, especially for irrigation in agriculture. In addition to the minimum requirements for the quality of recycled water, the regulation also stipulates the frequency of routine and validation monitoring. Somatic coliphages must be assessed as part of the validation monitoring of the device for the category with the most stringent water quality requirements (Class A, for which the following applies: “All food crops consumed raw, the edible part of which is in direct contact with recycled wastewater, and root crops consumed raw”) [2]. The purpose of validation monitoring is to evaluate the effectiveness of the device in removing selected indicator organisms of pathogenic bacteria, protozoa, and viruses.
Following the publication of the adopted legislative documents in the EU Official Journal, EU member states are subsequently obliged to incorporate the relevant changes into their legislation. EU regulations can be directly applied in EU member states. The method of determining somatic coliphages in water will therefore need to be introduced in some operational or other laboratories, and these laboratories will then have to devote some time to optimize the method.
The possibility of using the cultivation procedure in accordance with the procedure in the standard ČSN EN ISO 10705-2 [3] has been systematically dealt with since 2019 in the microbiological laboratory of the Department of Water Technology and Environmental Engineering at the University of Chemistry and Technology in Prague. The method introduced by this standard is used for the detection of somatic coliphages in differently loaded waters. When testing the determination procedure according to the said standard, the possibilities and possible applications of the method specified by the standard [3] for routine microbiological monitoring of the state of microbial contamination (or fecal contamination) of surface waters, wastewater (effluent from wastewater treatment plants), and grey water were identified. For interest, drinking water was also included in the tests. Concentration methods that must be used for water samples with a low incidence of somatic coliphages (< 3 PFU/ml) and are listed in the standard ČSN ISO 10705-3 [4] (valid from 1 December 2020) were tested and verified experimentally. Determination procedures and problems associated with the methods specified in the standard ČSN EN ISO 10705-2 [3] and in the standard for concentration methods ČSN ISO 10705-3 [4] are discussed further in the paper.
Methodology
In order to determine somatic coliphages, samples were taken of surface water, effluents from WWTPs, grey water, and drinking water. Somatic coliphages were subsequently determined according to the procedure specified in the standard ČSN EN ISO 10705-2 [3], Part 2: Quantitative determination of somatic coliphages. Due to the fact that the method is more suitable for waters with a higher incidence of somatic coliphages, selected concentration methods proposed in Annex A of the standard ČSN ISO 10705-3 [4], Part 2: Validation of methods for concentration of bacteriophages from water (method of membrane filtration and flocculation with magnesium hydroxide) were verified experimentally.
Procedure for determining somatic coliphages
The method of determination according to ČSN EN ISO 10705-2 [3] consists of cultivating and maintaining the appropriate host strain of E. coli, which is subsequently used for the determination of somatic coliphages by two-layer plaque titration. For testing purposes, the E. coli host strain was obtained from the collection of the State Institute of Public Health in Prague, under the designation CNCTC 5005. From the obtained lyophilized reference host strain, a stock and working culture was gradually prepared using modified Scholtens medium (MSB). The required density (108 CFU/ml) of working culture cells was checked spectrophotometrically by absorbance measurements prior to somatic coliphage determination, based on a pre-calibration between absorbance and number of colonies grown on modified MSA Scholtens medium.
The determination of somatic coliphages per se was subsequently performed according to the standard procedure given in the standard [3] with minor modifications. To temper the molten semi-liquid Scholtens medium (ssMSA) with the addition of calcium chloride, a 45 °C water bath was used. In total 2.5 ml of ssMSA medium, 1 ml of inoculum culture of the E. coli host strain, and 1 ml of the analysed sample were sequentially pipetted into sterile bacteriological tubes placed in a water bath. The mixture was stirred and subsequently poured onto the surface of the complete MSA medium in a Petri dish. After solidification, the dishes were incubated in a suspended position at 37 °C for about 18 hours. Somatic coliphages form visible zones of brightness on the surface of the medium, so-called plaques, which are read and subsequently expressed as the number of plaques forming units (PFU) in a unit of volume. To check the correctness of the procedure, a blank determination was also performed, which is a mixture of an inoculum culture of the host strain and a semi-liquid ssMSA medium.
Concentration methods
The efficiencies of selected concentration methods, in this case, membrane filtration and flocculation with magnesium hydroxide, were experimentally tested on real wastewater samples taken from effluent from the municipal mechanical-biological wastewater treatment plant (WWTP). Both methods were tested by the standard addition method. To determine flocculation efficiency, 10 ml of the WWTP effluent sample was dosed in 90 ml of standing tap water. In the case of membrane filtration testing, 1 ml (or 10 ml) of a sample of the effluent from the WWTP was pipetted into 100 ml (or 90 ml) of distilled water. In the WWTP effluent sample, i.e., in the sample for addition, somatic coliphages were determined in parallel according to the standard ČSN EN ISO 10705-2 [3]. The suspensions thus prepared were subsequently pre-treated by individual concentration methods to capture coliphages.
The method of flocculation with magnesium hydroxide consists of the separation of coliphages captured in the resulting flakes. Solutions of magnesium chloride (1 mol/l) and potassium hydrogen phosphate (1 mol/l) are gradually added to the tested volume of the prepared sample, at the volume specified in the standard ČSN ISO 10705-3 [4]. Sodium hydroxide solution (2 mol/l) is added dropwise until visible turbidity is obtained, while simultaneously checking the pH value, which should not exceed 8.6. The suspension is stirred slowly for about 15 minutes, during which the resulting flakes settle for about 30 minutes. After carefully withdrawing the supernatant, the thin sediment is concentrated by low speed centrifugation (relative centrifugal acceleration RCF = 1,000 g) for 15 minutes. After decanting the supernatant, the sediment is resuspended in peptone water with sodium chloride and vigorous shaking. Determination of somatic coliphages is then performed according to the standard ČSN EN ISO 10705-2 [3]. The effect of pH on flocculation (pH: 7.6; 8.0; 8.3; 8.6, and 9.0) was also monitored to test the effectiveness of the concentration method.
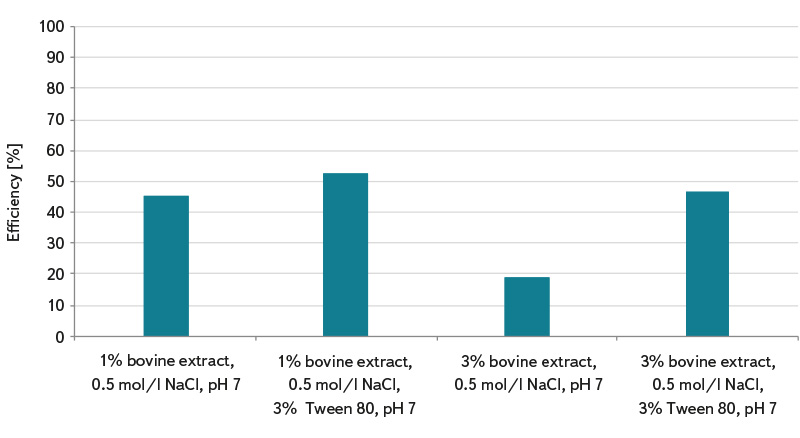
The membrane filtration method consists of filtering a given volume of sample through an electronegative filter made of a mixture of cellulose acetate and cellulose nitrate, with a pore size of 0.22 μm and a diameter of 47 mm. Filtration is preceded by the addition of magnesium chloride solution. The filter is then cut into about 8 smaller parts and placed in a glass flask with an elution solution (1% bovine extract, 0.5 mol/l NaCl, 3% Tween 80), which is placed in an ultrasonic bath for about 4 minutes. Simultaneously, the somatic coliphages released into the elution solution and collected on the filters are determined. As part of testing the effectiveness of the method, the effect of the composition of the elution solution was also monitored (here: 1% bovine extract, 0.5 mol/l NaCl; 1% bovine extract, 0.5 mol/l NaCl, 3% Tween 80; 3% bovine extract, 0.5 mol/l NaCl; 3% bovine extract, 0.5 mol/l NaCl, 3% Tween 80).
Results and discussion
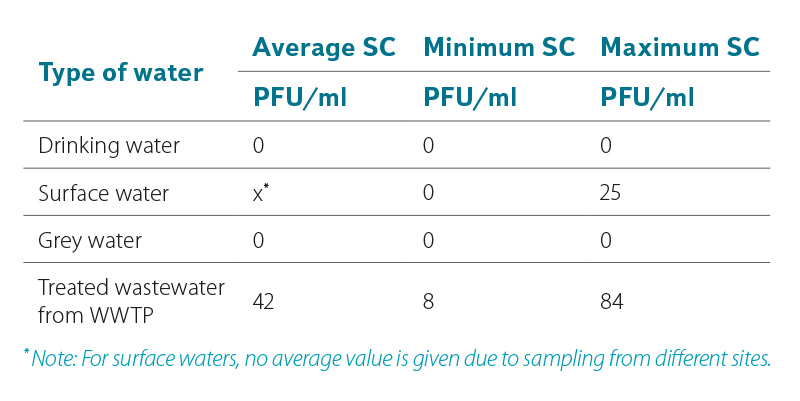
The presence of somatic coliphages in the aquatic environment usually indicates pollution caused by an animal or human feces. Although they are natural host strains of somatic coliphages of E. coli bacterium, some studies have found that they may use other related bacterial species that are not of fecal origin for their replication [5]. Comparison of the occurrence of somatic coliphages in differently loaded waters, or in drinking water, surface water, grey water, and in treated wastewater (effluent from WWTP) is evident in Table 1. Determination was performed according to the procedure specified in the standard ČSN EN ISO 10705-2 [3]. In the tested surface waters, the numbers of somatic coliphages ranged from 0 to 25 PFU/ml. Due to the fact that different types of surface water (flowing, standing) from different sites were taken and analysed, the average value of the number of PFU is not given. Of the tested waters, the highest numbers of somatic coliphages were found in effluent from wastewater treatment plants. A total of 6 different WWTPs were tested, while the findings of somatic coliphages in the effluent ranged from 8 to 84 PFU/ml with an average value of about 42 PFU/ml. Laboratory somatic coliphages have not yet been detected in the wastewater at the WWTP inflow (the reason is the increased occurrence of the accompanying microflora, which disturbs the plaque reading during evaluation). For the sake of completeness, the professional literature reports the numbers of somatic coliphages on inflows to WWTP in the range from 5·106 PFU/100 ml to 5·107 PFU/100 ml [6]. In grey water samples, in which the occurrence of coliform bacteria and possibly E. coli is common and was confirmed in the analysed samples (see Table 1), the presence of somatic coliphages was not confirmed by cultivation. As expected, the presence of somatic coliphages was not detected in drinking water taken from the consumer’s tap.
Table 1. Occurrence of somatic coliphages in various types of waters – procedure according to the standard ČSN EN ISO 10705-2 [3]
Due to the fact that the determination of somatic coliphages according to the standard ČSN EN ISO 10705-2 [3] uses 1 ml of sample for analysis, this procedure can lead to error and incorrect interpretation of test results in the analysis of waters with lower coliphage concentration and lower microbial contamination. Waters with less microbial activity (e.g. surface standing and running water, grey water, and drinking water) require pre-treatment of the sample by the method of their concentration according to ČSN ISO 10705-3 [4]. The use of a reliable method of concentrating somatic coliphages in a water sample before its analysis can significantly improve the detection efficiency. The graphs in Fig. 1 to 3 show the laboratory-determined efficiencies of concentration methods of magnesium hydroxide flocculation and membrane filtration. In the magnesium hydroxide flocculation method, the concentration efficiency of somatic coliphages ranged from about 32% to 64%, and its pH dependence was found. The highest numbers of somatic coliphages were determined for samples with pH 8.0 (concentration efficiency about 64%) and 8.3 (concentration efficiency about 64%), see Fig. 1. It can be assumed that the efficiency of capturing coliphages in flakes will be higher when concentrating more organically loaded water. The membrane filtration method showed significantly higher efficiency compared to flocculation. Depending on the elution solution used, efficiency ranged from about 40% to 100%, see Fig. 2. The content of the non-ionic surfactant Tween 80 in the elution solution is essential for the release of somatic coliphages from the membrane filters. With its help, about 100% efficiency of the concentration of somatic coliphages was achieved in most cases. As can be seen in Fig. 3, magnesium chloride (which is added to the sample before it is filtered) is also important in the process of concentrating by membrane filtration. Without its use, average capture efficiencies of somatic coliphages ranged from 19% to 52%, depending on the elution solution. Multivalent cations such as Mg2+, Ca2+, and Al3+ allow more efficient adsorption of coliphages on electronegative membrane filters at lower pH [7].
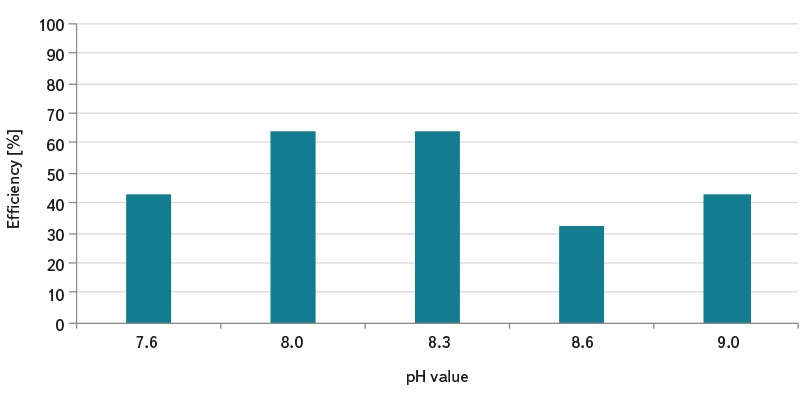
Fig. 1. Magnesium hydroxide flocculation efficiency in the concentration of somatic coliphages and the effect of pH
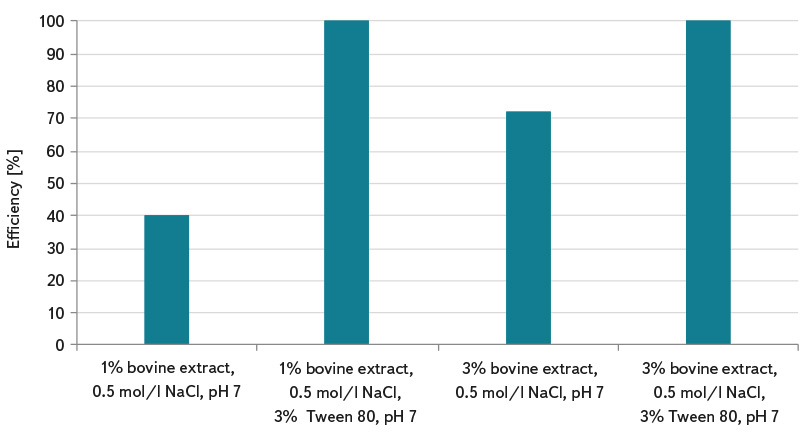
Fig. 2. Membrane filtration efficiency according to the procedure in ČSN ISO 10705-3 [4] (including the addition of MgCl2) in the concentration of somatic coliphages and the effect of different elution solution
Fig. 3. Membrane filtration efficiency according to the procedure in ČSN ISO 10705-3 [4] (without addition of MgCl2) in the concentration of somatic coliphages and the effect of different elution solution
Problems in the determination of somatic coliphages in waters
In this presented case, the determination of somatic coliphages was performed, except for a few minor modifications, according to the valid standard ČSN EN ISO 10705-2 [3]. These minor modifications include, for example, the order of application of the individual components to the bacteriological tubes. Initially, mainly due to the rapid solidification (lumping) of the semi-liquid ssMSA medium, it was preferred to initially apply 1 ml of the test sample, 1 ml of inoculum culture, and only subsequently to add 2.5 ml of ssMSA medium. However, using control blanks, it has been found that initial application of the sample to bacteriological tubes can subsequently cross-contaminate the inoculum culture and the ssMSA medium with coliphages. Thus, application in the following order was preferred: semi-liquid ssMSA medium, inoculum culture, test sample. The solution to the rapid solidification of the semi-liquid ssMSA medium can be either the addition of a lower amount of agar (with the risk of the top layer sliding) or the choice of agar with a lower solidification temperature. Alternatively, the test sample can be applied to the bacteriological tubes in such a way that it slowly runs down the tempered wall of the tube. The prepared mixture was always lightly stirred by hand to prevent the formation of air bubbles and quickly poured onto the complete MSA medium in Petri dishes. Evaluation, or the reading of the formed plaques of somatic coliphages, is disturbed not only by the presence of air bubbles, but also by the presence of condensed water adhering to the surface of the solidified upper layer (even in the suspended position). For this reason, the dishes should first be pre-dried with the lids partially open in a laboratory thermostat before incubation (this is stated in the standard ČSN EN ISO 10705-2 [3] and it is important).
During the experimental verification of selected concentration methods described in the standard ČSN ISO 10705-3 [4], some problematic procedures were also found. In the case of the magnesium hydroxide flocculation method, sedimentation of all formed flakes did not occur visibly. To capture as many as possible, it was necessary to centrifuge a larger volume of sample, or 50 ml tubes were used. Resuspension of the resulting sediment in peptone water containing NaCl can be problematic in less than 30 ml; in some cases it was not possible to dissolve the flakes either by vigorous shaking or by vortexing. In the case of a sample with a low concentration of coliphages (< 3 PFU/ml) which is concentrated by flocculation, it would be appropriate to work simultaneously with its two different volumes, at least one of which will allow quantification of plaques in 1 ml (or 5 ml) of total volume of 30 ml.
Compared to flocculation, the membrane filtration method is significantly faster and simpler. The most problematic in this case is working with filters. After filtration of the sample, individual filters should be cut into about 8 parts, treated in an ultrasonic bath, and subsequently, applied face down to the layer of ssMSA medium with the host strain. Simultaneously with the filters, the elution solution should be determined by the method according to ČSN EN ISO 10705-2 [3]. Not only does this work with filters require a certain skill with tweezers, but it does not bring the expected results, or somatic coliphages in the form of plaques cannot be read with certainty from cultivated plates, see Fig. 4.
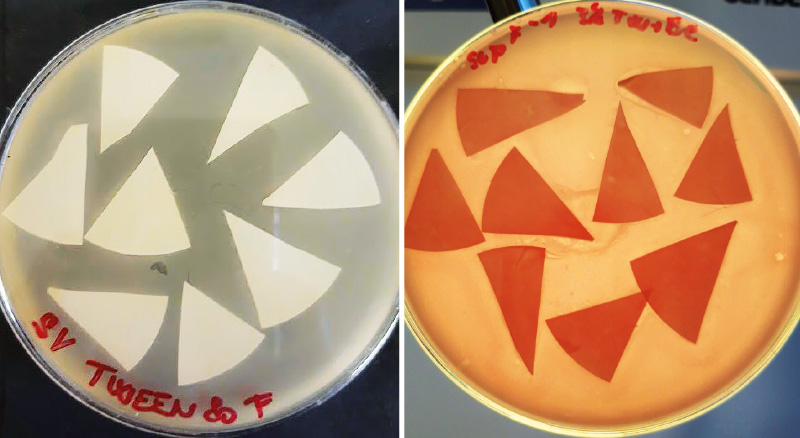
Fig. 4. Determination of somatic coliphages using membrane filtration – problematic enumeration of captured coliphages on filters
Conclusion
The indication of fecal contamination is an important tool for determining the safety of water. Recently, attention has shifted to other possible indicator organisms, specifically somatic coliphages, which can be used to monitor the effectiveness of removing small and more resistant particles (e.g. viruses) by water treatment or water purification. As part of tests performed in the microbiological laboratory of the Department of Water Technology and Environmental Engineering at the University of Chemistry and Technology in Prague, it was found that the procedure for determining somatic coliphages according to ČSN EN ISO 10705-2 [3] is not suitable for drinking water, grey water, and some surface waters; that is, where a very low or zero concentration of coliphages is expected. For these types of waters, in most cases it will be necessary to use the concentration methods specified in ČSN ISO 10705-3 [4], which will convert somatic coliphages from a sample with a larger volume to a smaller volume. Methods are selected based on sample volume, particle content or turbidity. From the experience gained so far with the method of magnesium hydroxide flocculation and membrane filtration on electronegative filters, it can be said that the latter method is able to more efficiently concentrate somatic coliphages from a water sample. In addition to being relatively fast and less space-consuming, it is also likely to be more feasible for operations laboratories.
Acknowledgments
This output was created within the project of Specific University Research – project No. A2_FTOP_2020_026.