SUMMARY
Rapid Small-Scale Column Tests (RSSCTs) can be used for quick and simple laboratory verification of the adsorption efficiencies of micropollutants on various adsorbents. The selection of adsorbent is the most important aspect when incorporating adsorption technology into the operation of a drinking water purification plant or a wastewater treatment plant. The tests are carried out at a laboratory-scale, which minimizes costs for materials and operation. This article focuses on the adsorption on granular activated carbon (GAC), as it is the most commonly used type of adsorbent. However, other types of adsorbents can be used for RSSCTs. The aim of this article is to introduce the reader to the issue of RSSCTs. The article specifically states the possible purposes of using RSSCTs and defines important parameters in the field of micropollutant adsorption testing on activated carbon which is applied in the dimensioning of tests and in their subsequent evaluation. The article also provides summary information on test methodology. The analysis of mostly foreign literature reveals that RSSCTs methodologies in individual studies show various modifications; however, essentially it is the same procedure. The differences are evident at the level of material equipment, construction dimensions, and setting parameter values; this is mostly due to different purposes of testing.
INTRODUCTION
Rapid Small-Scale Column Tests (RSSCTs) are tests that examine the adsorption of micropollutants on a selected adsorbent. The principle of the method is the flow rate of test water through a small column filled with adsorbent, which allows more representative data to be obtained than in batch tests [1]. RSSCTs methodology was developed in the 1980s [2] as a scaled-down version of pilot testing in large granular activated carbon (GAC) columns [3]. RSSCTs are advantageous for minimizing time and economic demands compared to field tests. Their use significantly reduces the amount of material required for construction of the columns, amount of adsorbent, volume of water, and test operating time [4]. GAC is mostly used as an adsorbent, mainly due to its excellent adsorption capabilities and its wide availability on the market. However, other adsorbents can also be tested.
Other options can be chosen for testing micropollutant sorption on GAC, including pilot testing, which takes place in large columns. The difference compared to RSSCTs is that pilot tests can be used directly at the site of interest and testing usually takes place over a long period of time. Another possibility is mathematical and statistical predictive models [2]. Unlike predictive models, RSSCTs do not require extensive isothermal or kinetic studies [3].
RSSCTs can be used for many purposes. Previous studies with RSSCTs have focused on testing different adsorption media to remove a specific element from water (e.g., arsenic adsorption [5]), a specific organic substance (MTBE adsorption [6], geosmin adsorption [7]), or a mixture of substances (adsorption of pharmaceuticals and their metabolites [8], PFAS adsorption [9]). Other useful information can be found at a smaller laboratory scale. RSSCTs can be used, for example, to examine the effect of operating parameters on adsorption efficiency, such as EBCT (Empty Bed Contact Time), or they can be used to obtain breakthrough curves of individual micropollutants [5, 10]. Breakthrough curves are a graphical representation of the dependence of the monitored micropollutant concentration on the effluent from the column to the micropollutant concentration on the inflow to the column per unit of time [11]. Using RSSCTs, breakthrough curves can be obtained in a fraction of the time compared to large-scale testing [4]. RSSCTs are often the most appropriate way to evaluate different types of GAC for a given site (especially specific treated water). They can also quickly verify the manufacturer’s claims and get an idea of the economic costs of adsorption and select the appropriate type of GAC for pilot testing [3, 10].
Accurate prediction of GAC effectiveness is particularly important for selecting the appropriate type of GAC for both field studies and real operation [12]. The parameters commonly reported by activated carbon manufacturers, such as iodine value, dechlorination half-value, BET isotherm, etc., give little indication of how a given type of GAC will behave in real conditions and to what extent the specific micropollutants will bind to it [6]. Therefore, it is advisable to test the type first in the laboratory. A constant or proportional diffusivity model is used for subsequent conversion from laboratory scale to field and operational filter size [2]. Using constant diffusivity design, we assume that diffusivity of the adsorbed substance is independent of adsorbent particle size [6, 8, 9]. Using the proportional diffusivity equation, on the other hand, we assume that intraparticle diffusivity is linearly dependent on adsorbent particle size [2, 13]. Computer models can be used to determine parameter values and breakthrough curves, e.g., FAST 2.0 software [8].
Important parameters
In testing adsorption on activated carbon, as well as on other adsorbents, there are important parameters that must be taken into account when dimensioning the test. These parameters also facilitate interpretation of results and allow comparison of results from different studies. The most commonly used include EBCT, flow, and HLR, but other parameters may be useful as well. Below are some selected ones [8, 9,13]:
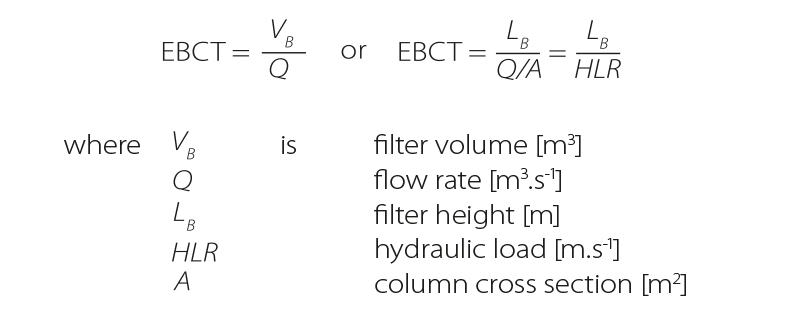
- Empty Bed Contact Time EBCTThe EBCT indicator is defined as the time for which flowing water is retained in the column volume occupied by GAC. EBCT is given in minutes or seconds and depends on the height of the GAC layer and selected filtration rate.
The EBCT value is based on filter size design as well as estimated amount of GAC used. The EBCT value also affects breakthrough curves of the monitored pollutant, which may affect GAC service life. Higher EBCT values, i.e., higher contact time (retention), generally increase adsorption efficiency.
- Flow Rate Q
The flow rate, i.e., volume of water that flows through the column per unit of time, can be calculated (in addition to the basic formula), for example, by deriving it from the EBCT or HLR equation. The flow rate is most often stated in m3.s-1 or m3.day-1. However, on a reduced rapid test scale, it is commonly stated in ml.min-1.
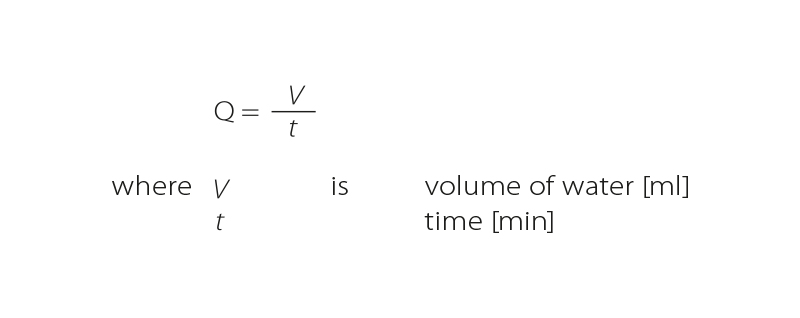
- Hydraulic Loading Rate HLR
Another important characteristic is hydraulic loading rate, i.e., the flow rate divided by the area of the column [10]. The term Filter Velocities (vF), which corresponds to HLR, also appears in some literature. The unit m.h-1 and then cm.min-1 are most often used for this parameter.
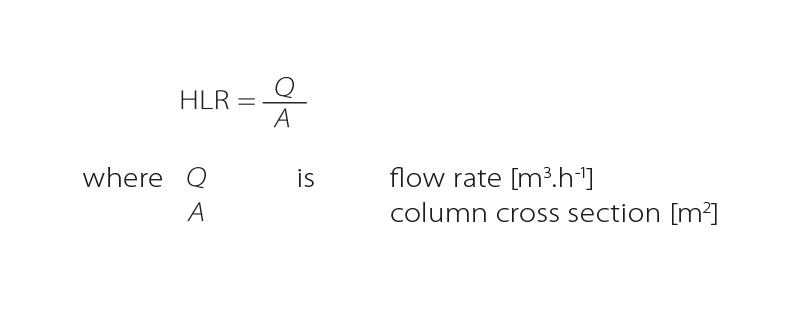
- Filter Operation Time tF
This indicates the time from the start of the test to its end or to replacement of the activated carbon by regenerated or new activated carbon. The parameter is mainly used for longer-term tests on a larger scale and is usually stated in days. - Throughput Volume VL
This is the volume of water that passes through the column during filter operation. The parameter is mainly used for longer-term tests on a larger scale.

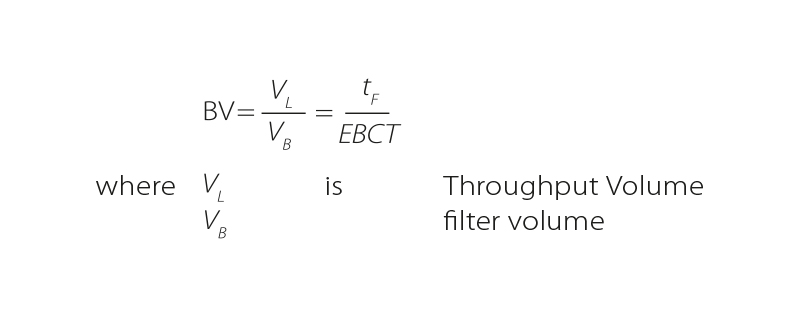
- Bed Volume BV
This parameter is dimensionless and is a standardization of the volume of water flowing to the volume of the activated carbon bed. It can be expressed mathematically as the ratio of the volume of water flowed to the volume of GAC in the column (filter volume). However, it can also be defined as the ratio between test operating time and EBCT.
- CUR (Carbon Usage Rate)
This indicator defines GAC service life. It can be understood as the need for a certain amount of activated carbon in order to reach the target concentration for the monitored micropollutant. The unit is kg.m-3.
RSSCTs METHODOLOGY IN THE LITERATURE
The studies performed are diverse in terms of research purpose, which is also reflected in differences in methodology. However, it is basically a similar procedure – the flow of test water through the column. They differ from each other mainly in the subject of testing, design dimensions, amount of adsorbent used, or parameter values [5].
Model water can be chosen for testing, i.e. an artificially prepared solution with a known concentration of test substance, which will decrease by passing through the columns. Model water is useful in understanding certain basic adsorption relationships [6]. The use of actual drinking or wastewater allows assessment of adsorbent suitability for specific future uses. When using wastewater, it is advisable to first remove undissolved substances in order to avoid clogging of the adsorbent in the column. After mechanical and biological treatment, wastewater can be, for example, filtered through an ultrafiltration membrane [15]; however, standard laboratory filter paper will suffice.
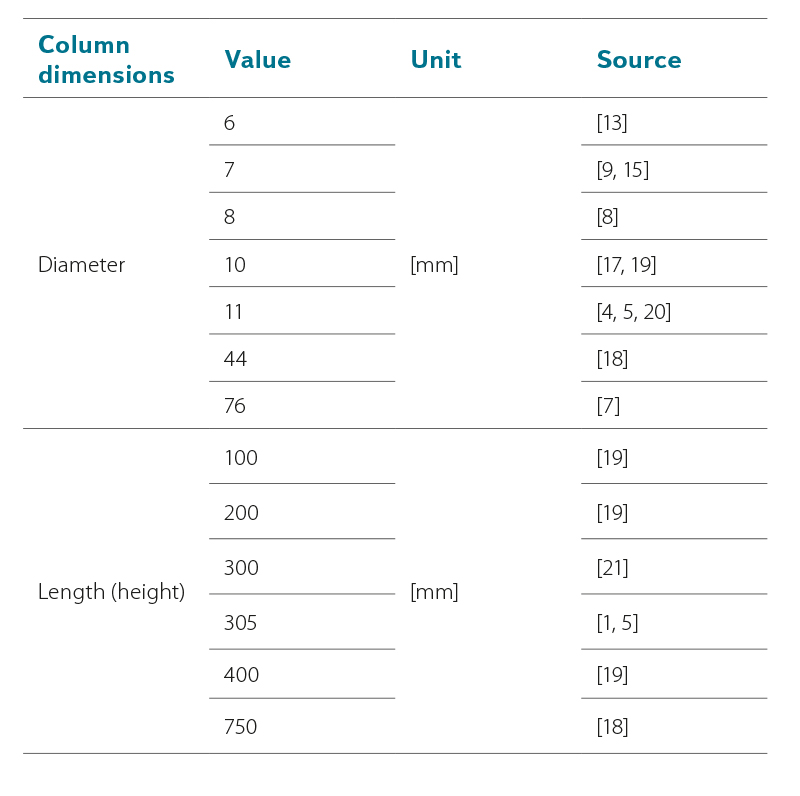
The preparation of test columns is a basic step in RSSCTs preparation. The number of columns selected and their size, as well as the number of test repeats, reflect the purpose and scope of intended testing. Borosilicate glass is often used to make columns. Column dimensions vary greatly from study to study. Inside diameter ranges from 6 mm to 76 mm, and column length (height) from 100 to 750 mm. In some studies, only column diameter is shown. An overview of column dimensions is shown in Tab. 1.
Tab. 1. Column dimensions reported in RSSCTs studies
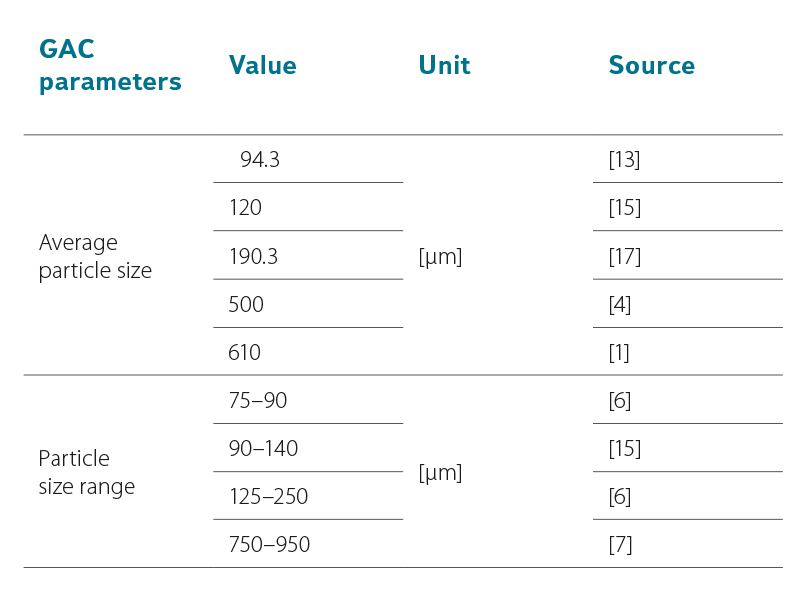
The size of GAC fraction was also different in each study. Activated carbon can be used without treatment, or it can be crushed, ground, and then sieved to form a fraction of the desired size [5, 15, 16]. For example, a ball grinder can be used for grinding. GAC fractionation can be achieved using several nets with different mesh sizes [17]. After crushing, it is advisable to wash the fraction to remove excess dust, and then dry the GAC (at 105° C) and store it in a desiccator until use [1, 17]. GAC can be used without additional modification as supplied by the manufacturer [18]. In some studies, one average GAC fraction size is reported [1, 4, 15], in others a range of sizes is used to possibly increase adsorption rate [6, 7, 15]. Average particle size can be determined using a light microscope [17]. Details of GAC particle size occurring in the available literature are shown in Tab. 2.
Tab. 2. GAC parameters shown in RSSCTs studies
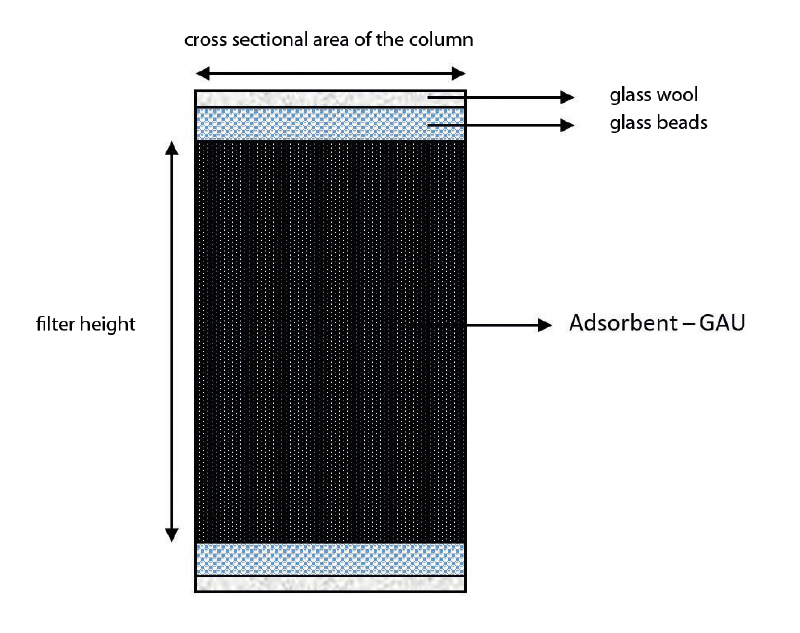
In addition to the adsorbent itself, the column is filled with other components. The most common method in the studies is filling the glass column with a layer of glass balls and glass wool above and below the bed with GAC (Fig. 1, 2). Glass balls (1–3 mm) and glass wool are mainly used to fix activated carbon [1, 5, 8, 15]. In some studies, fine sand (0.45–0.55 mm particles) [7], glass balls of different diameters [16], or glass balls supplemented with a membrane PFTE filter (80–120 µm) [17] are used for a similar purpose. Ezzati et al. [19] report that they washed glass wool with acid before the columns were constructed to prevent microbial growth. Other necessary equipment are suitable column closures, hoses for conducting the test water into the column, and throttle clamps for regulating the outflow from the column and a stand for the entire apparatus. The columns can be mounted either in a laboratory stand, or in a specially designed stand [2].
 Fig. 1. Scheme of filled glass column (author: Anna Kólová)
Fig. 1. Scheme of filled glass column (author: Anna Kólová)
 Fig. 2. Photo of glass column filled with GAC during the testing (author: Anna Kólová)
Fig. 2. Photo of glass column filled with GAC during the testing (author: Anna Kólová)
Prior to the actual test, it is advisable to first rinse the assembled and sealed glass column containing GAC with distilled or deionized water (at least 15 minutes) to detect any leaks and design problems. This step also allows the medium to be compacted and incorporated inside the column [5]. In their study, Cantoni et al. [9] had the columns incorporated with deionized water for two hours. However, most studies report shorter periods. It has also been suggested that GAC in columns should be incorporated directly with the test water [2]. However, the incorporation of GAC can also be done in another way, e.g. according to the recommendation of the manufacturer of a specific GAC [18].
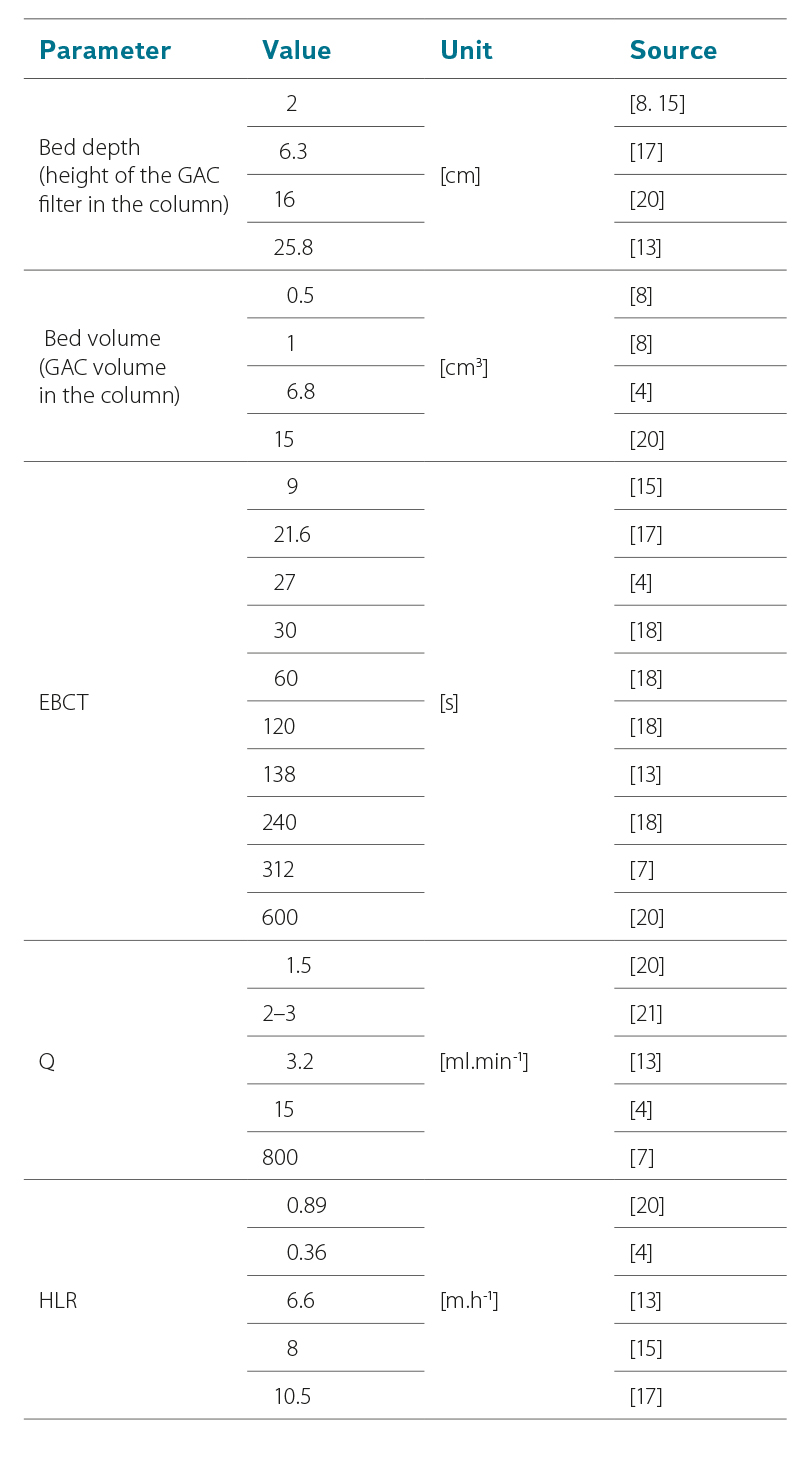
Test water can flow freely through the column by gravity; however, more often the water is driven by a pump [11]. Piston and peristaltic pumps are suitable for RSSCTs, which usually use lower flow rates. The values of the achieved parameters, including flow rates, are shown in Tab. 3. The values of the parameters, amount of GAC used, and design dimensions of the columns are interdependent. A very important parameter is EBCT, which some studies developed in more detail and examined its effect on the adsorption of various micropollutants (by including several EBCT values) [6, 18].
Tab. 3. Values of selected parameters shown in studies on RSSCTs
The method and frequency of sampling depends on the length and purpose of the test. In some studies, automatic samplers were used for sampling [9, 15]. In addition to concentrations of monitored pollutants, it is appropriate to determine some basic chemical indicators, such as A254, pH, COD [2], or to monitor the turbidity or temperature of samples [18] to further evaluate efficiency of adsorption on GAC.
RSSCTs usually last several hours; however, there are also cases where the test time was set for several days [9]. If the tests take longer, columns can be wrapped in an opaque material (e.g., aluminium foil) or placed in the dark to prevent negative effects of light, e.g. photodegradation of micropollutants or growth of microorganisms and algae [6, 13]. Long-term tests lose the advantage of test speed; however, reduced material and water consumption costs are still significant.
CONCLUSION
RSSCTs are a suitable tool for rapidly obtaining results in the field of adsorption of micropollutants on a specific adsorption medium. They can be used for many different purposes, e.g. to initially verify the effectiveness of a particular GAC to remove micropollutants from water before its subsequent long-term use on a field scale, or to research adsorption principles. The great advantage of rapid tests is the saving of time and costs for the construction and operation of columns.
This article can serve as a guide to design one’s own RSSCTs methodology. When designing it, the purpose of testing must be taken into account, from which will be derived the selected matrix (model or real water), adsorbent, test operation mode, number of columns and their filling (adsorbent, glass balls, glass wool), setting test parameters (Q, EBCT, HLR, amount of adsorbent), test operation time, sampling frequency, etc. As the studies analysis shows, rapid tests show considerable variability of performances and can be adapted to the particular research purpose and possibilities.
Acknowledgements
The article was written thanks to support from the Institutional Funds for the Development of a Research Organization –TGM WRI, p. r. i., within the internal grant “Testing of carbon nanotubes using rapid small-scale column tests” (3600.52.13/2021).
The article has been peer-reviewed.